Non-invasive diagnostics
PANEL
OWLiver®
OWLiver® Panel: A non-invasive diagnostic tool for assessing all lesional phases of MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease). This lipidomic analysis of fasting blood samples uses high-resolution liquid chromatography coupled with mass spectrometry (UHPLC-MS) to measure a panel of lipid biomarkers reflecting liver fat content, inflammation, and fibrosis.
Non-invasive diagnostics
About
OWLiver®
Developed by Rubió Metabolomics, S.L.U., the OWLiver® Panel is a CE-marked in vitro diagnostic medical device, complying with Directive 98/79/EC. Its three algorithms includes lipid concentrations and clinical data, providing an estimation of MASLD stage based on a multicenter, multiethnic study involving patients affected by different severity of type 2 diabetes mellitus and a body mass index above 25 kg/m2.
Progression of Hepatic Disease
Interactive by OWL Metabolomics by Rubió
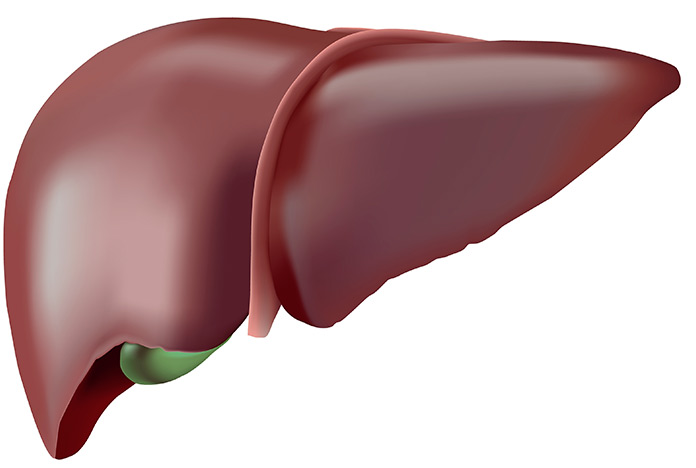
HEALTHY LIVER
Less than 5% of liver cells contain fat.Reddish brown color with uniform texture.
Average weight between 1400 and 1800 grams.
In addition to fat, inflammation and liver cell damage, scar tissue increases.
Fibrosis leads to loss of elasticity and liver function.
It significantly increases the risk of death from cardiovascular disease.
Between 10-15% of people with steatohepatitis progress to cirrhosis.
In patients with F4 there is a high risk of developing end-stage liver diseases, such as liver cancer.
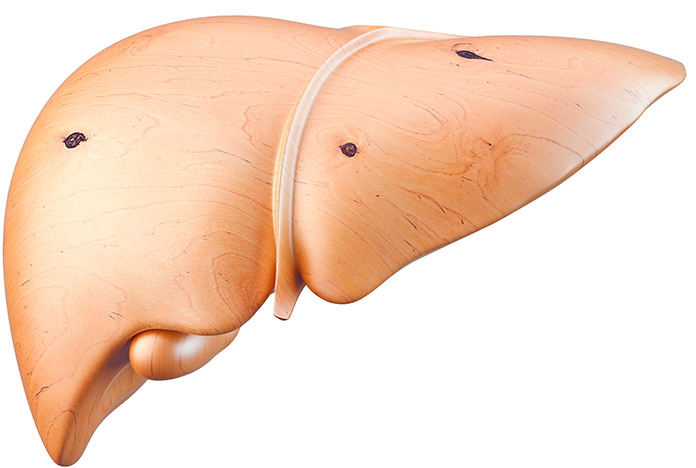
HEPATIC STEATOSIS
More than 5% of liver cells contain fat.The liver takes on a mottled, soft, pale yellow appearance and increases in size.
Approximately 25-30% of the world’s population has hepatic steatosis.
In people with obesity or type 2 diabetes mellitus, the incidence increases to 70%.
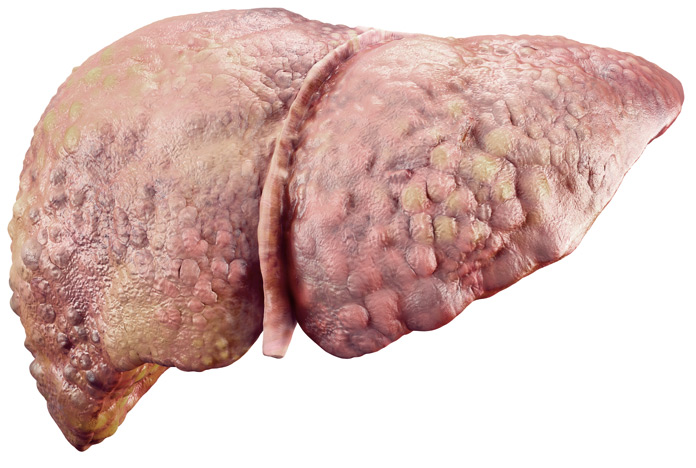
STEATOHEPATITIS WITH FIBROSIS (F0-F1)
Between 15-20% of people with hepatic steatosis develop steatohepatitis.Inflammatory infiltrates and liver cell damage (ballooning) are observed.
Fibrosis or scarring begins to appear.
The risk of death from cardiovascular disease increases.
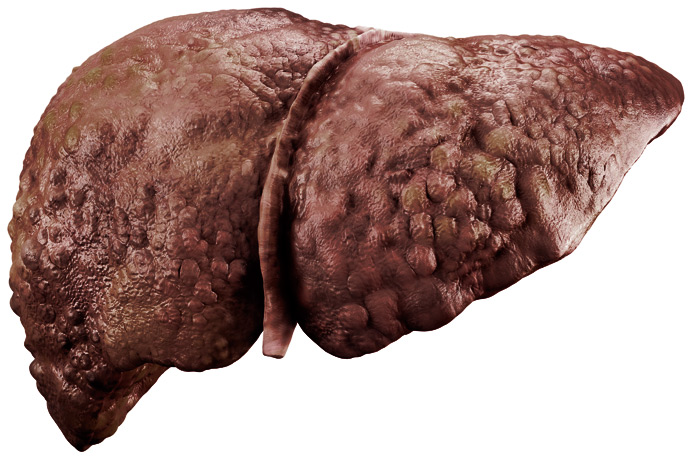
STEATOHEPATITIS WITH FIBROSIS (F≥2)
The liver is significantly reduced in size and appears brownish in color.In addition to fat, inflammation and liver cell damage, scar tissue increases.
Fibrosis leads to loss of elasticity and liver function.
It significantly increases the risk of death from cardiovascular disease.
Between 10-15% of people with steatohepatitis progress to cirrhosis.
In patients with F4 there is a high risk of developing end-stage liver diseases, such as liver cancer.